The Great Periodic Table
Too many chemical elements!
Or not enough? Either way we all know they are needed and vital for life.
In this blog article we plan to explain what the periodic table is and also what the chemical elements in it are together with chemical properties etc.
We will also give you a valuable new source to practice your knowledge of the periodic table.
Can’t wait to the end of this article? Then head to the practice test section where you have 100 new test questions on this topic.
So, what is the periodic table?
The periodic table is a way to see visually the chemical behaviour of various chemical elements and what their properties are. With the help of the table you can see what chemical elements are similar and behave similarly but the table goes a lot further than that as well and allows chemists to even predict properties of new chemical elements yet to be discovered or synthesized.
How is this possible you might ask? We can’t know something exists before we know it exists!
Well with the help of the periodic table we can, and chemists and scientists have been able to predict elements in the past which have later been found to have the chemical properties described by the scientists even before they even had a name for the element.
Who came up with this thing?
The first widely documented periodic table was published in 1869 by a well-known Russian chemist by the name of Dmitri Mendeleev. You can read more about this interesting man here.
He wanted to show that the chemical elements known back then had similarities and periodic trends. He even made some predictions of elements not yet known but that he believed would be found some day.
Was he right?
You bet he was! Most of his predictions were actually proven to be correct.
Details please!
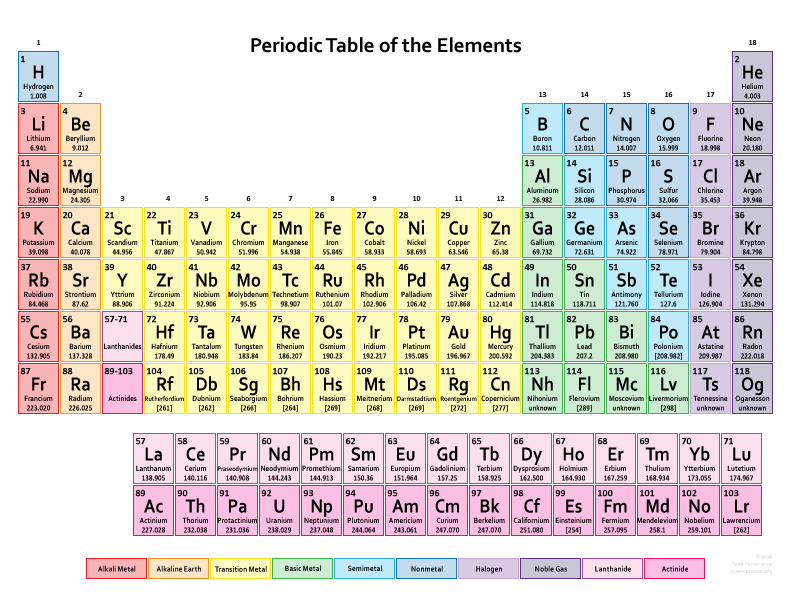
Interested in more details? Well, each of the chemical elements in the periodic table has a unique atomic number. This number represents the number of protons in its nucleus.
As Wikipedia puts it: “Most chemical elements have varying numbers of neutrons among the different atoms, and these variants are being referred to as isotopes. Isotopes are never separated in the periodic table; and instead they are always grouped together under a single element. In turn elements with no stable isotopes have the atomic masses of their most stable isotopes, where such masses are shown, listed in parentheses.”
There are 118 elements in the periodic table and new ones are still being added. In fact in 2016, 4 new elements were added which are tennessine (Ts), nihonium (Nh), moscovium (Mc) and oganesson (Og).
About the elements though, the first 94 of them actually occur naturally whereas the remaining ones only occur in laboratory conditions when synthesized.

About the table itself
The vertical columns in the table are called groups or family. Elements in the same group tend to have shared chemistry and similar properties.
The horizontal rows in the table are called periods. Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity.
In the table the elements can all be classified into 2 major categories which are metals, non-metals and metalloids.
As a general rule remember this:
- Metals are solid and conductive.
- Non-metals are mostly gases.
- Metalloids are elements which have mixed properties of the 2 mentioned above.
Now each of the can be divided into further smaller categories but we will not go into that much depth here.
How to use the periodic table
The table contains a wide amount of information. You can see from most tables the atomic mass, atomic number, element symbols, how the element possible behaves in relation to similar elements and what kind of element it is in the grand scheme.
The symbols for each element comes normally from a simple abbreviation from the name but sometimes the Latin name is used as well.
The atomic number again is a deciding factor in separating one element from another. The modern periodic table is in fact organized in order of increasing atomic number.
The atomic mass of the elements is measured in atomic mass units.
Last thing to know is that many periodic tables nowadays also identify element types using different colours. These include for example alkali metals, basic metals, semimetals, alkaline earths, transition metals, lanthanides, non-metals, actinides, halogens and noble gases.
The way the periodic table has been organised or laid out is so that it leads to periodic table trends.
These are:
Ionization Energy – this is the energy needed to remove an electron from a gaseous atom. Ionization energy increases when moving left to right in the table and decreases when moving down an element group.
Electronegativity – this tells us how likely an atom is to form a chemical bond. Electronegativity increases when moving left to right in the periodic table and decreases moving down a group.
Atomic Radius – this is a measure of the size of the atom and in the table the atomic radius decreases while moving left to right across a row and increases when moving down a group.
Electron Affinity – this just tells us how readily an atom accepts an electron. Electron affinity increases when moving across a period and decreases moving down a group. It is also good to know that the electron affinity is almost zero for noble gases.
More on this can be found here as well.
How does all this affect me in any way?
Well that my friend is a tough statement to make and the answer is in EVERY POSSIBLE WAY!
There is nothing in this world that doesn’t have to do with the periodic table.
Take hydrogen for example, it is the building block of life itself and makes up 90% of the atoms in the universe. Helium again can be found in the stars and lasers and balloons. Lithium is used a medicine but also in batteries. Carbon again makes up life itself and diamonds for all the ladies out there reading this!
The list of uses would be a whole different article altogether but there is a good place where you can read more about the uses. Check out this site for that.
Recap
So, what have your learnt from this blog article and can take away? We to start we went through what the periodic table is and the history of the table itself. We then went in more detail though the table and why it was put together the way it was.
We also explained how to use the periodic element table and what uses it has together with various chemical elements in it. All highly useful for everyday life let me assure you.
Last but not least we promised to give you a place to practice your knowledge and improve and you can do it with these 100 chemistry test questions which have been added to our site. They will help you in your preparation we guarantee.
More educational and fun tests below.


